Research Interests
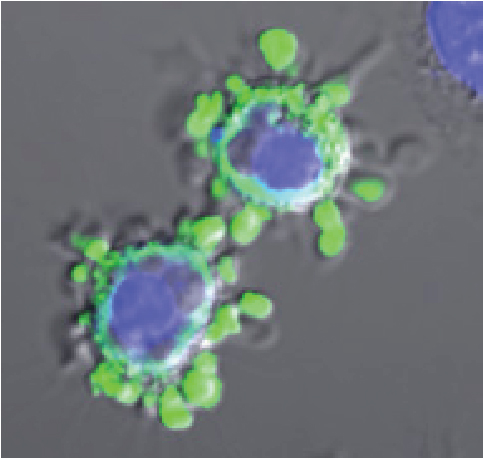
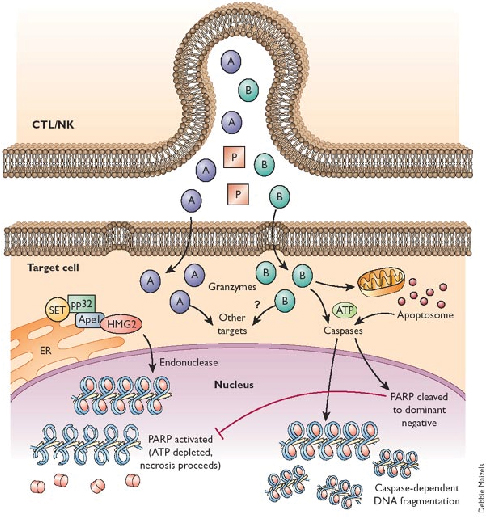
The primary focus of my lab is the study of cell death and how this impacts on inflammation. We are especially interested in the natural cell death process known as apoptosis (programmed cell death), as well as unprogrammed cell death (necrosis). We are exploring how necrosis can promote activation of the immune system (i.e. promote inflammation), through release of IL-1 family cytokines (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β, IL-36γ) and how apoptosis can also modulate inflammation. A major focus of the lab at present is understanding how cell stress (in the form of diverse chemotherapeutic agents) initiates inflammation and how this impacts upon therapeutic outcomes
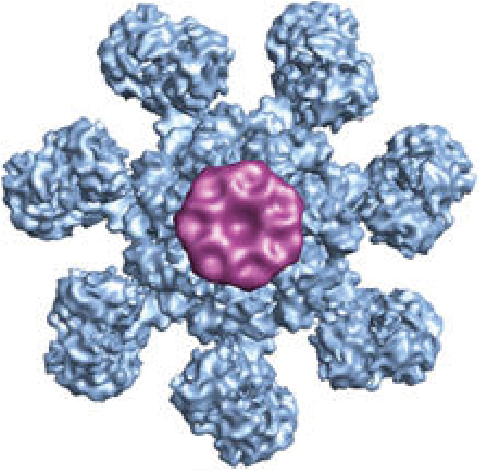
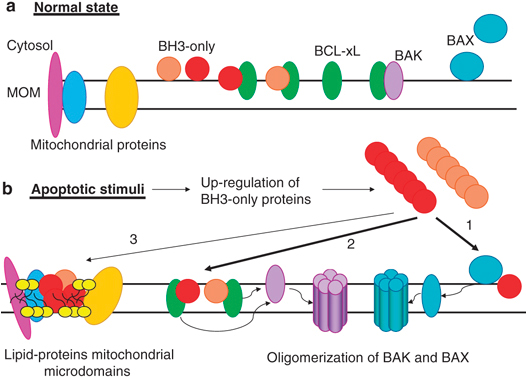
Apoptosis is a mode of cell death that is under molecular control and can be triggered by a multitude of stimuli - both physiological as well as pathological. Cells die by apoptosis during development, tissue homeostasis, fine tuning of the immune system, and due to the normal wear and tear that multicellular organisms experience in everyday life. Apoptosis is also observed as a part of the damage-limitation response seen during infectious disease and is seen during many other pathological conditions, such as cancer and neurodegeneration.
Understanding how and why cells die, at a molecular level, is leading to new insights into many fundamental biological processes as well as new ways of treating conditions where either too few (cancer, autoimmune disease) or too many (neurodegeneration) cells die.
See our publications page for examples of our work in these areas.