

|
| Home | Research | Publications | Recent news | Group | Teaching |
|
n-type transparent conducting oxide researchDefect engineering in PbO2The high conductivity, abundance, and inexpense of β-PbO2 have led to its global application as the active material in the cathode of lead-acid batteries, although the fundamental reasons for this conductivity and the nature of the defects involved have long proven to be contentious issues in the literature. The hybrid-DFT calculations carried out here not only elucidate the nature of the band gap and identify the dominant defects in PbO2 as oxygen vacancies, but also utilise this comprehensive analysis of the electronic structure to predict a novel potential application of the material: as a narrow band gap TCO. Narrow band gap TCOs are highly conductive materials with fundamental band gaps far smaller than 3.1 eV but sufficiently large optical band gaps due to lower energy transitions being symmetry forbidden. It is predicted that PbO2 can be made into a narrow band gap TCO via control of the carrier concentration, such that a window of transparency occurs in which both the effective optical band gap and intraband transitions are greater than 3.1 eV despite a direct band gap of just 0.35 eV. This is theoretically achieved in PbO2 with a carrier concentration in the range 9.14×1020–1.07×1021 cm-3, which through the process of a Moss-Burstein shift, renders the material transparent.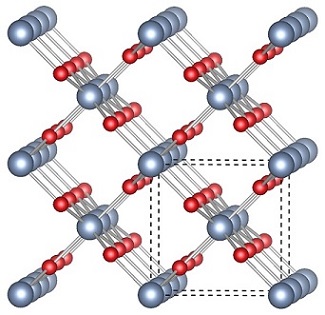 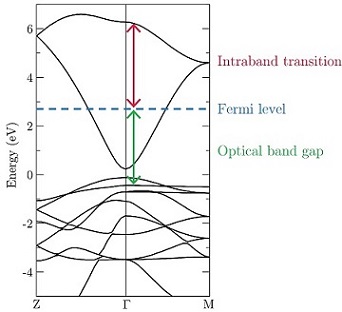 Related references:
Small polarons in Nb- and Ta-doped rutile and anatase TiO2Experimentally, Nb- and Ta-doping of anatase TiO2 have been reported to increase the n-type conductivity by four orders of magnitude, making n-type doped anatase an excellent non-toxic, cost-efficient candidate as a TCO. Nb- and Ta-doping of rutile TiO2, however, does not increase the conductivity by such a large amount, and has been experimentally characterized as a polaronic semiconductor, not a degenerate semiconductor like doped anatase. The difference in conducting behaviour between these two closely related polymorphs is somewhat surprising, and therefore we have investigated the electronic structure of Nb- and Ta-doped anatase and rutile TiO2 using first principles methods. We find localized Ti3+ states in the band gap of both materials when Nb/Ta-doped, indicating the formation of small polarons in both polymorphs. On this basis we suggest the experimental variance between polymorphs in doped thin films is not an inherent property of the bulk crystals but is due to other factors, e.g. additional defects or sample morphology, dependent on the synthesis history. For both anatase and rutile the defect feature is found to be insensitive to the identity of the dopant, and similar Ti3+ polarons are expected generally for doping where electrons are donated to the Ti lattice. 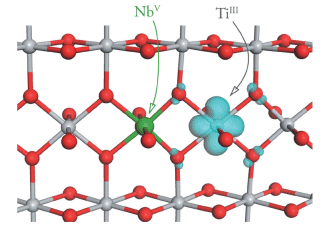 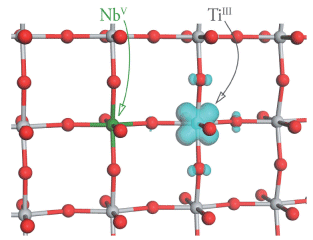 Related references:
Intrinsic defects in SnO2SnO2 is known to behave as an n-type transparent conducting oxide in its oxygen deficient form. However, the exact cause of oxygen deficiency is not well understood, with some controversy over whether it is a result of oxygen vacancies or tin interstitials. Conductivity occurs through the introduction of shallow defect levels located 0.15-0.30 eV below the conduction band minimum which introduce electrons into the conduction band. Through the use of density functional theory calculations as implemented in VASP, we have examined the energetics of a number of defects in tin dioxide under both oxygen-poor and oxygen-rich conditions. Stoichiometric Frenkel and Schottky defects were found to have very high formation energies under all conditions which is the reason for the observed non-stoichiometry of the material. Under oxygen-poor conditions, the most stable intrinsic defect and hence the likely cause of oxygen deficiency was found to be oxygen vacancies (VO//) charge-compensated by the reduction of Sn(IV) ions neighbouring the vacancy to Sn(II) (SnSn//). These are likely to lead to n-type conductivity through the mobility of electrons from Sn(IV) to Sn(II) sites. Although extreme oxygen-rich conditions were found to result in a low formation energy for oxygen interstitials, these relax to form peroxide ions (O2 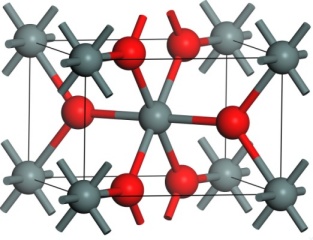 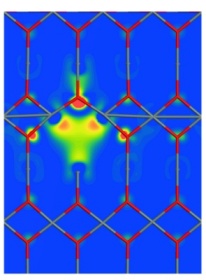 Related references:
Email: watsong AT tcd.ie Last updated: Apr 14 2012 Back to Top |